%%{init: {'theme': 'base', 'themeVariables': { 'primaryColor': '#f0f0f0', 'edgeLabelBackground':'#ffffff', 'tertiaryColor': '#f0f0f0'}}}%%
flowchart TD
A[Start Application] --> B[Select Section 21 Application]
B --> C[Select Medicine Category]
C --> D[Choose Patient Type]
D --> E{Patient Type?}
E -->|Named Patient| F[Complete Named Patient Form]
E -->|Multiple Patient| G[Complete Multiple Patient Form]
F --> H[Fill Applicant Details]
G --> H
H --> I[Fill Importer Information]
I --> J[Fill Medicine Details]
J --> K[Process Payment]
K --> L[Application Submitted]
Overview
What are Section 21 Applications?
Section 21 of the Medicines and Related Substances Act allows for the use of unregistered medicines in South Africa under specific circumstances. The Section 21 application process enables healthcare professionals to request authorization from SAHPRA to access and use medicines that:
- Are not registered in South Africa
- Are registered but are being used for an indication not included in the approved Professional Information
- Are registered but are being used at a different dosage or via a different route than approved
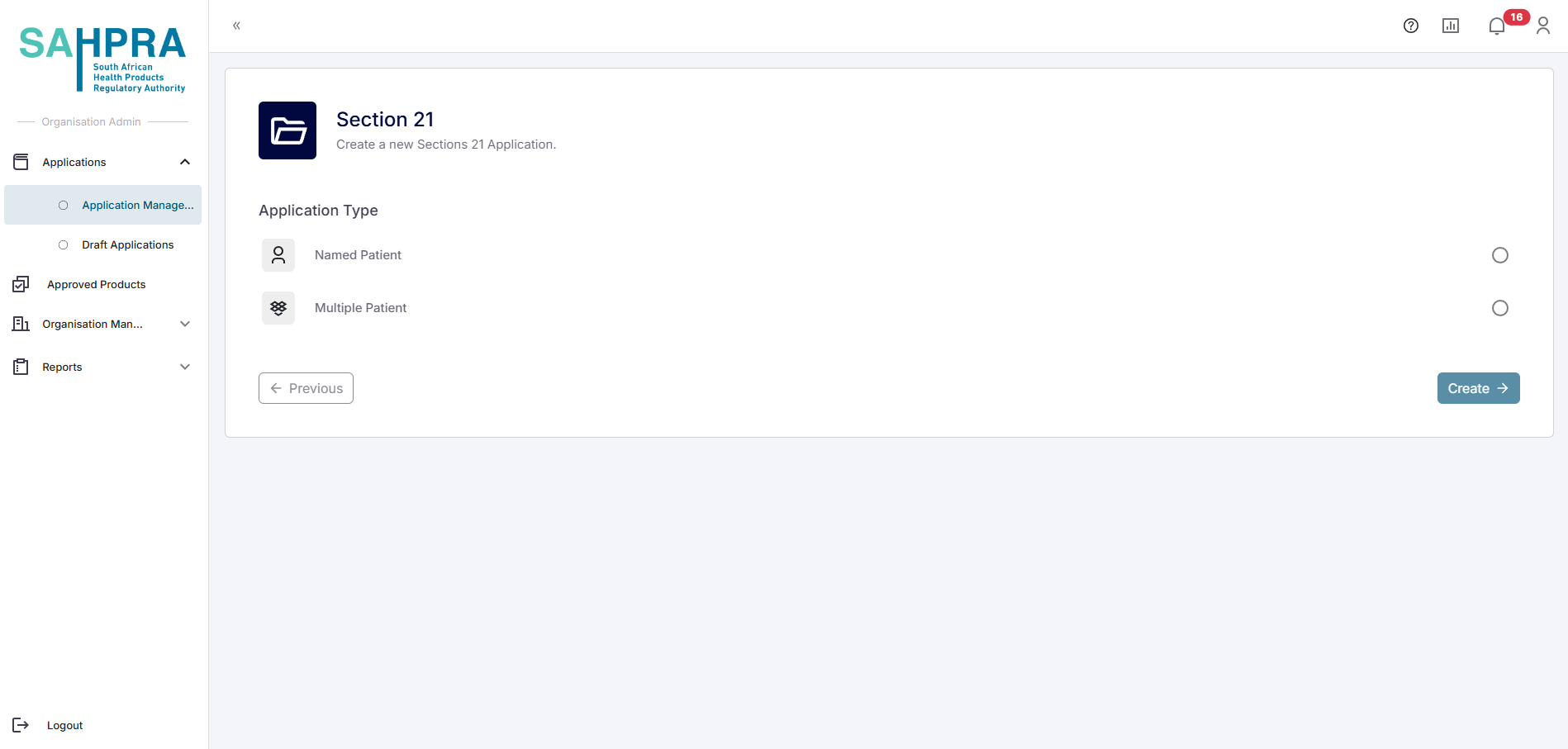
Types of Section 21 Applications
There are two main types of Section 21 applications:
- Named Patient Applications: For individual patients requiring unregistered medicine
- Multiple Patient Applications: For groups of patients requiring the same unregistered medicine
Application Process Overview
Key Information Required
Regardless of the application type, you will need to provide:
1. Applicant Information
- Professional registration details
- Qualification and specialty
- Contact information
2. Importer Information
- Licensed importer details
- SAHPRA license number
- SMF number
- Importer address
3. Patient Information
For Named Patient applications: - Patient demographics - Diagnosis details (ICD10 codes) - Clinical information
For Multiple Patient applications: - General diagnosis information - Estimated number of patients
4. Medicine Information
- API manufacturer details
- Final product manufacturer information
- GMP information
- Drug details (name, strength, dosage)
- Registration status in other countries
5. Supporting Documentation
- Patient consent forms (for Named Patient applications)
- Professional registration certificates
- Scientific rationale for using the unregistered medicine
- Product information from other regulatory authorities
Fees and Payment
Section 21 applications require payment of the prescribed fee. The payment process is similar to other SAHPRA applications:
- Submit the application
- View the order details
- Make payment via EFT
- Upload proof of payment
- Wait for payment verification
Processing Timeframes
- Named Patient applications are typically processed within 24-48 hours
- Multiple Patient applications may take longer, depending on complexity
- Applications cannot be processed until payment is verified
Next Steps
- For Named Patient applications, proceed to Named Patient Applications
- For Multiple Patient applications, proceed to Multiple Patient Applications
